About vanadium
Vanadium is a medium-hard, steel-blue metal. Although a lesser-known metal, it is quite valuable in the manufacturing industry due to its malleable, ductile and corrosion-resistant qualities.
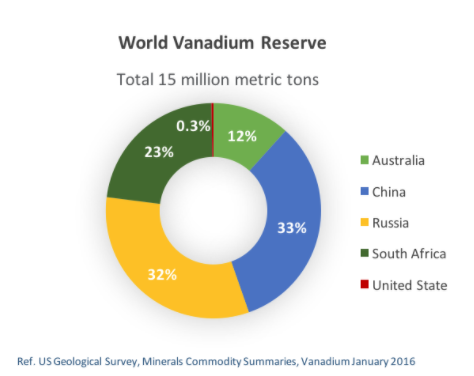
Vanadium rarely exists as a free element in nature but can be found in about 65 different minerals, including magnetite, vanadinite, carnotite and patronite. It also can be found in phosphate rock and some crude oils. Vanadium is usually obtained by heating crushed ore in the presence of carbon and chlorine to produce vanadium trichloride, which is then heated with magnesium in an argon atmosphere, according to Jefferson Lab. Around 98 percent of mined vanadium ore comes from South Africa, Russia, and China.
Vanadium makes up 150 parts per million (ppm) of the Earth's core and comprises 0.019 percent of the Earth's crust, according to PeriodicTable.com. The cosmic quantity of vanadium in the universe is about 0.0001 percent. Vanadium can be detected spectroscopically in the Sun's rays and occasionally in the light of other stars. y
Vanadium was discovered twice.

01
History of Vanadium
How was Vanadium discovered, and by who?
The element vanadium has an interesting history and was even discovered twice. In 1794, a Spanish-Mexican mineralogist, Andrés Manuel Del Río, was offered the chair of mineralogy at the Royal School of Mines in Mexico City. Here, he was responsible for analyzing the chemistry of new minerals. In 1801, he found a brown lead ore from a mine named La Purísima del Cardenal, resulting in his discovery of a new element, which we would know as vanadium. However, at the time, Del Río called his element panchromium, meaning “all of the colors” because of the element’s colorful salts. He later renamed the element erythronium, after the Greek word, “eruthros”, meaning red, because Group 1 and Group 2 oxide salts of the element would turn red when heated.
A few years later, in 1805, French chemist Hippolyte-Victor Collet-Descotils examined the same brown lead ore and stated that erythronium was actually impure chromium. Unfortunately, Del Río agreed that he was wrong about his finding. It was not until 1830 when a Swedish chemist named Nils Gabriel Sefström found an iron ore and discovered an element. He named this “new” element vanadium, after ‘Vanadis’, the Scandinavian goddess of beauty. Not long after, German chemist Friedrich Wöhler analyzed Del Río’s brown lead ore with Sefström’s iron ore and realized that the two “different” elements were actually identical.
02
Physical Properties of the Element Vanadium
- Melting point: 2183 K; 1910°C; 3470°F
- Boiling point: 3680 K; 3407°C; 6165°F
- Density: 6.0 g/cm3
- Atomic Symbol: V
- Atomic weight: 50.942
- Atomic number: 23
- Electronegativity: 1.63
- Classification: Transition metal
- Natural abundance in the Earth’s crust: 0.019%
- Electron shell configuration: [Ar] 3d3 4s2
- Isotopes: Vanadium-50 and vanadium-51 are naturally occurring
- Found naturally in the minerals: Vanadium is found in 65 different minerals, including vanadinite, carnotite, and magnetite
- Toxicity: Non-toxic to humans at normal concentrations, but its compounds can be toxic
Major deposits of vanadium
Vanadium reserves
03
Vanadium mining & production
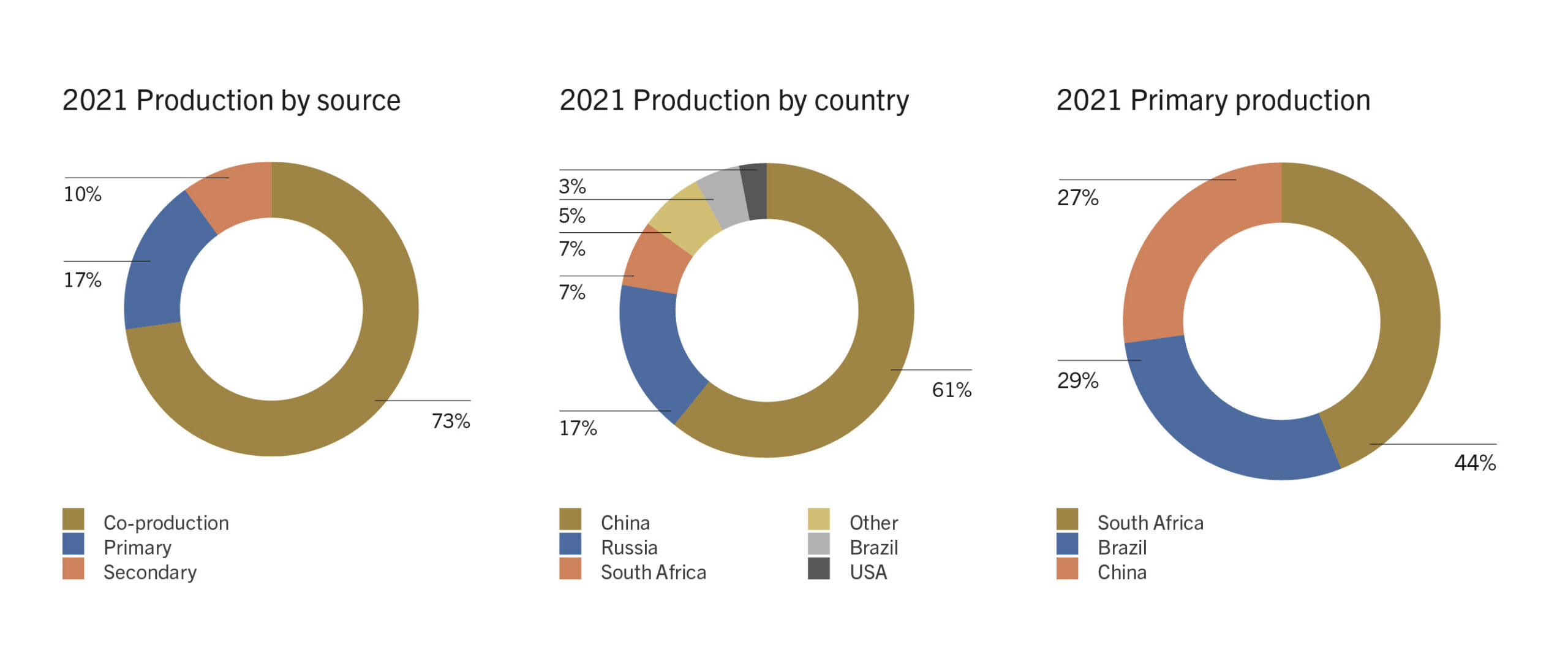
Vanadium from the titaniferous magnetite ores of Russia and China and South Africa is extracted as a co-product in steelmaking. In China and Russia the iron is produced in a blast furnace but in South Africa the iron is produced by a process involving the pre-reduction of the magnetite with powdered coal in a rotary kiln followed by reduction in a submerged arc electric furnace. Vanadium bearing coproduct slag is generated during steel production from iron sands in New Zealand in a process similar to the South African process.
The iron from these operations contains about 1.5% vanadium which is removed as slag by low temperature treatment with oxygen. In China this is carried out by spray refining, while in South Africa it is done in a shaking ladle and in Russia a special oxygen steel converter is used.
The slag from South Africa contains up to 25% V2O5 whereas the slag from China and Russia contains between 14% and 22%. The V2O5 is extracted from the slags by a roast-leach process in which the slags are roasted in kilns or in multi-hearth furnaces with sodium carbonate, chloride or sulphate (or lime in Russia). This produces sodium vanadates which are leached into an aqueous phase with water. Ammonium vanadates are precipitated from this solution by addition of ammonia and sulphuric acid to control the pH.
The ammonia is removed and the vanadate converted to various oxides by heating under controlled conditions which are varied according to the oxide required. Fused flake V2O5 is produced by decomposing the vanadates in a furnace and melting the resulting V2O5 to liquid phase and then casting onto a chilling wheel. Vanadium oxide powders are produced by the solid state decomposition of the vanadates in a controlled environment. In some cases liquid-liquid ion exchange/solvent extraction processes are utilized to produce high purity vanadium oxide powders.
Vanadium oxides are used for the production of ferrovanadium and vanadium-aluminium alloys required for the addition of vanadium to steel and titanium respectively. Ammonium vanadates and high purity vanadium oxide powders are used for the production of downstream vanadium chemicals.
Vanadium from the Colorado carnotite ore is extracted as a co-product during uranium production. The ore is treated with sulphuric acid to dissolve the vanadium and uranium. The uranium and vanadium are separated from the liquid by solvent extraction followed by a liquid-liquid ion exchange process which separates the uranium leaving the vanadium in the acid solution. This is subsequently oxidised and removed from the organic salts with soda ash. Vanadium polyvanadate is precipitated by the addition ammonium sulphate.
Vanadium, from oils in which it is present, is obtained by various routes. It is present in the coke produced in the Flexicoke process used in Venezuela for the upgrading of heavy crude oils. Vanadium from this coke leached into solution with sulphuric acid and ammonium vanadates are precipitated.
Vanadium bearing oil based fuels are burnt in the boilers of electric power generating plant and vanadium is left in the fly ash and boiler slags. Vanadium in these ashes and slags is recovered by the same process as for vanadium coming from Flexicoke. Ashes and slag are also converted directly to ferrovanadium (40%V grade) via silicon reduction in an electric furnace.
Spent nickel-molybdenum and cobalt-molybdenum catalysts are treated pyrometallurgically to solubilise the metals present (V, Co, Ni, Mo), separate the metals and precipitate them as salts (including vanadates) before converting to oxides.
Vanadium is added to steel as a ferroalloy. Ferrovanadium is available in alloys containing 40%, 60% or 80% vanadium. The 60% and 80% grades are mostly produced by the aluminothermic reduction of vanadium oxides in the presence of steel scrap or by direct reduction in an electric furnace. The 40% grade is produced from slag and other vanadium products by the silicon reduction process.
Vanadium additions to titanium alloys are made with aluminium-vanadium master alloys which are also produced by aluminothermic reduction of high purity vanadium oxides.
04
Uses
Around 80 percent of the vanadium produced is alloyed with iron to make a shock- and corrosion-resistant steel additive called ferrovanadium, according to Jefferson Lab. Ferrovanadium contains between 1 to 6 percent vanadium.
Vanadium-steel alloys are used to make extremely tough tools such as axles, armor plates, car gears, springs, cutting tools, piston rods and crankshafts. Vanadium alloys are also used to make nuclear reactors because of their low-neutron-absorbing properties, according to the Royal Society of Chemistry. In fact, the first widespread industrial use for vanadium was in the steel framework of the Model T Ford, which allowed for a lighter weight frame that was also of greater tensile strength.
The compound vanadium pentoxide (V2O5) is used as a mordant (a substance that permanently fixes dyes to fabrics), as a catalyst in some chemical reactions and in the manufacturing of ceramics. It can also be combined with gallium to form superconductive magnets, according to Jefferson Lab. When mixed with aluminum and titanium, vanadium can create a very strong alloy that is used for special applications such as dental implants and jet engines.
05
Vanadium Reactions
Vanadium is a moderately reactive element that acts as both a metal and nonmetal in certain reactions. It becomes more reactive at elevated temperatures. In normal conditions, vanadium is unaffected by oxygen and water because of its protective oxide layer. It is also resistant to molten alkali metal.
When heated, vanadium metal reacts with excess oxygen in the air to form vanadium(V) oxide, V2O5. Vanadium metal is quite reactive at elevated temperatures, resulting in the possibility of the vanadium(V) oxide containing other vanadium oxides as well. Lower oxidation states of vanadium may re-oxidize, until no longer exposed to oxygen.
It is only reactive with certain acids, which act as oxidizing agents. When vanadium(II) combines with an acid, it produces blue-colored dioxovanadium(V), VO2+, ions. When ammonium metavanadate, NH4VO3 combines with hot hydrochloric acid, it forms a reddish-brown chloro-complex and reduces the compound to a +4 oxidation state.
Fluorine, F2, a halogen, will react with vanadium at elevated temperatures. When fluorine and vanadium react, it produces the colorless vanadium(V) fluoride. This compound is extremely reactive and will vaporize easily.
06
Vanadium Compounds
There are several compounds that vanadium exists in. They present themselves in a wide range of colors, explaining why the element was named after the Scandinavian goddess of beauty.
Oxides
Vanadium oxides play a large role in the industrial field for their resistance to corrosion and rust. Their primary use is in steel alloys, called ferrovanadium alloys. At temperatures above 660˚C (1220˚F), vanadium oxide metals become reactive and will readily oxidize. Interestingly, each oxidation state associates itself with a specific color.
Vanadium(II)
Vanadium monoxide, VO is an electronically neutral reagent. It comes as a grey metallic solid and is an electrical conductor with a high melting point of 1789˚C (3252.2˚F). Its solution is a purple color that oxidizes to vanadium(III) when exposed to air, changing the color to green. Additionally, adding nitric acid to vanadium(II) solution can produce blue colored vanadyl ions, VO2+.
Vanadium(III)
Vanadium trioxide, V2O3, is a black, crystalline solid that is slightly soluble in water. This basic oxide occurs naturally in karelianite mineral. Additionally, it functions as a reducing agent. When exposed to air, vanadium trioxide oxides decompose to the blue colored vanadium(IV) state.
Vanadium(IV)
Vanadium dioxide, VO2, is amphoteric, meaning it can behave as both a base and acid. When put into an acid solution, vanadium dioxide dissociates into blue-colored vanadyl ions, VO2+. In a base solution, vanadium dioxide produces yellowish-brown colored hypovanadate ions, [V4O9]2-.
Vanadium(V)
Vanadium pentoxide, V2O5, is vanadium’s most important compound because of its thermal stability and abundance. It has a density of 3.35 g/cm3 and is not very soluble in water. This yellow to red crystalline powder has several functions, including being a mordant, acting as a catalyst for some chemical reactions, being useful in the production of ceramics, and forming superconductive magnets with gallium. Despite its widespread applications, this compound can be toxic when ingested, inhaled, or in contact with skin. Ammonium metavanadate, NH4VO3, a compound where vanadium presents itself in the +5 oxidation state, reduces under acidic conditions. Adding zinc and a moderately concentrated acid will reduce vanadium(V) to vanadium (IV).
Vanadium-containing minerals, like carnotite, are processed to isolate vanadium. First, the carnotite ore goes through a leaching process, where it gets treated with hot sulfuric acid and an oxidizing agent for 24 hours. The leaching process works to convert vanadium into a soluble salt. Notably, uranium is also found in carnotite, and therefore, uranium salts are also extracted. The salts then get filtered out and the remaining “ore” further processed. This isolates vanadium from uranium, allowing for vanadium to go through a precipitation reaction with ammonium sulfate. This precipitation reaction produces ammonium metavanadate. Lastly, ammonium metavanadate is filtered out and calcined to vanadium pentoxide.